Press Releases
|
|||||
Quell is a wearable, transcutaneous electrical nerve stimulation (TENS) device for knee, foot and leg pain that is available over-the-counter. It can be used during the day while active and at night while sleeping. Users can personalize and manage therapy discreetly via the mobile app for iPhone and Android smartphones. Quell is also a pain management solution with pain, sleep, activity and gait tracking. The Quell Health Cloud® provides customized feedback and powers one of the largest chronic pain outcomes databases. To learn more visit www.QuellRelief.com.
Like Quell, a smartwatch is always on the body. This enables a high level of integration and novel pain relief functionality. It is for this reason that
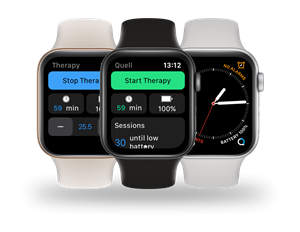
The Quell Watch app is standalone, so it functions independently of the iPhone. It gives a Quell user the ability to control the device and monitor pain relief from the wrist; a level of convenience never before achieved with a TENS device. The user simply taps the Quell complication to open the app. The user can then easily start or stop therapy, increase or decrease intensity, view their therapy schedule, and get Quell status information. The battery level can be checked by just glancing at the complication.
A particularly valuable feature of the Quell Watch app is notifications. The app is in regular, behind the scenes, communication with Quell. This enables alerts such as when the Quell battery is low or if therapy unexpectedly halted. These notifications help ensure that the user does not miss out on pain relief. The notifications are configurable so the Quell Watch app experience can be personalized.
"The promise of wearable technology is to enhance wellness and help manage disease by empowering people to monitor and improve their own health. Quell is the clear innovation leader in wearable pain relief," said
About
SVP and Chief Financial Officer
neurometrix.ir@neurometrix.com
Source:
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/98b63a9d-7a78-45e9-8852-350357f41c75
Source: NeuroMetrix, Inc.